Every day, premature babies are examined for retinopathy of prematurity (ROP) in the Neonatal Intensive Care Unit (NICU) at Children's Hospital Colorado. This is a condition that can cause blindness when abnormal blood vessels grow in the retina. The examination is essential, but also emotionally difficult: premature babies are vulnerable, must be restrained and may experience a temporary drop in breathing or heart rate during the examination. Many parents therefore choose to step away for a moment.
Imagine if AI could make these examinations faster, more consistent and less stressful. And imagine if AI could not only detect eye diseases, but also reveal systemic disorders via the retina, an emerging field of research known as oculomics. This is precisely what a team led by ophthalmologist Dr. Emily Cole and AI researcher Dr. Praveer Singh (University of Colorado Anschutz) is working on. Their goal was to develop AI models that add immediate clinical value in the NICU. ‘We are developing algorithms with real clinical applicability. Not just in the lab, but at the patient's bedside,’ says Singh.
Challenges of ROP screening
ROP occurs in premature babies when the blood vessels in the retina are still immature. In the oxygen-rich NICU environment, these vessels can grow abnormally, with a risk of bleeding, retinal detachment and permanent vision loss. Every year, approximately 14,000 babies in the US are diagnosed with ROP, of which 1,100 to 1,500 require treatment.
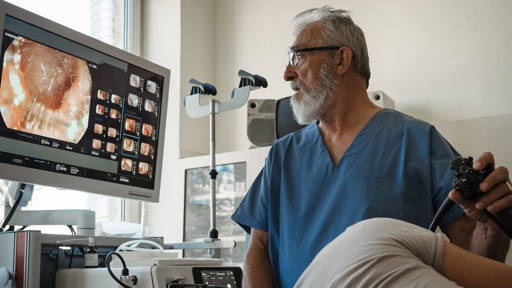
The examination is done using fundus photographs and a physical eye examination, in which a camera is placed on the eye. It is invasive, technically difficult and requires repetition due to a shortage of specialised doctors. In addition, the assessment of ROP varies from doctor to doctor, especially when estimating the degree to which blood vessels ‘twist’, which is the criterion for treatment decisions.
Faster and more objective assessment
AI can make a difference here. Whereas doctors classify ROP into three categories (plus / pre-plus / no plus), an AI model can analyse retinal images and quantify vascular tortuosity on a scale of 1 to 9. This vascular severity score makes interpretation uniform and supports decisions about follow-up examinations and treatment.
In 2022, Cole published studies confirming that AI can reduce variation between clinicians. A single image is sufficient for a risk score that is fast, reproducible and less dependent on individual assessment. ‘The score gives me immediate direction in the treatment plan. It creates a common language for all healthcare providers,’ says Cole.
Predicting other diseases in the future
Singh has been working on ROP AI for years and has successfully validated algorithms in multiple countries, even with different camera types. For NICUs without expensive imaging equipment, the team is investigating smartphone telescreening, which is cheaper and surprisingly accurate.
And the step forward is bigger than just ROP: recent studies show that retinal AI analysis may also be able to detect pulmonary hypertension (PH) and bronchopulmonary dysplasia (BPD) early, even before invasive diagnostics are needed. ‘We can detect conditions weeks earlier using AI than with catheterisation or ultrasound. This can identify at-risk babies more quickly and enable tailored care,’ says Singh.
Challenges for implementation
Cole is now focusing on implementation research: how does AI fit into the NICU workflow, which professionals should be involved, and how do we ensure support from neonatologists, nurses, and parents? Pilot implementations should demonstrate how AI screening speeds up research, reduces stress in babies, and improves access to care, including through telemonitoring and transfer moments.
‘Adoption by healthcare providers is crucial. We are not only developing this technically, but together with the users. Only then will it really work,’ says Cole. When AI becomes established in the NICU, it could become a new standard: faster screening, more objective assessment, earlier prediction of diseases and less stressful eye examinations for the smallest patients.
AquaWomb
Earlier this year we wrote about AquaWomb, a spin-off from Eindhoven University of Technology and originating from the Horizon project Perinatal Life Support. They are developing an innovative water incubator for extremely premature babies. In this artificial amniotic fluid environment, they can continue to develop before transitioning to lung breathing. The technology is intended for neonatal intensive care units and is being developed by Myrthe van der Ven, Guid Oei, Marcel Quist and TU/e.
The first development phase focuses on an advanced manikin of a 24-week foetus, equipped with sensors and linked to digital simulation models, so that testing is possible without direct use on patients. AquaWomb closely involves healthcare professionals and parents in the development and also uses the manikin as a training tool. The company is actively increasing its visibility through international hospitals. AquaWomb distinguishes itself through an obstetric approach in which the baby is placed in the incubator after a caesarean section via a transfer bag filled with artificial amniotic fluid. Thanks to digital simulations, a large part of the process can take place without animal testing.