A research team at Texas A&M University has developed a wearable biosensor that attaches to a urinary catheter bag and can detect urinary tract infections (UTIs) significantly earlier than standard laboratory diagnostics. Combined with a smartphone application, the system enables real-time monitoring and automated alerts. And thus potentially reducing complications, hospital stays and healthcare costs.
Urinary tract infections remain among the most common bacterial infections worldwide. In hospital settings, catheter-associated UTIs (CAUTIs) account for more than half of all healthcare-associated infections. Vulnerable populations, such as older adults, post-surgical patients and individuals with chronic conditions, are particularly at risk.
Accurate detection
Early and accurate detection is essential. When treated promptly, UTIs are generally manageable. However, delayed diagnosis can allow infections to progress, potentially leading to serious complications such as urosepsis.
Current diagnostic approaches present clear limitations. Urinalysis, often based on dipstick testing, offers rapid results but lacks sensitivity and may produce false negatives. Urine cultures, considered the gold standard, provide higher reliability but require up to 48 hours for definitive results. This diagnostic window can delay treatment and increase the risk of adverse outcomes. The findings were published in Biosensors and Bioelectronics.
Continuous E. coli monitoring
To address this gap, the Texas A&M team, led by Dr. Hatice Ceylan Koydemir, Associate Professor of Biomedical Engineering, developed a fluorescence-based sensing system designed for continuous monitoring. The wearable device adheres directly to a catheter bag and detects the presence of Escherichia coli (E. coli), the bacterium most frequently responsible for catheter-associated UTIs.
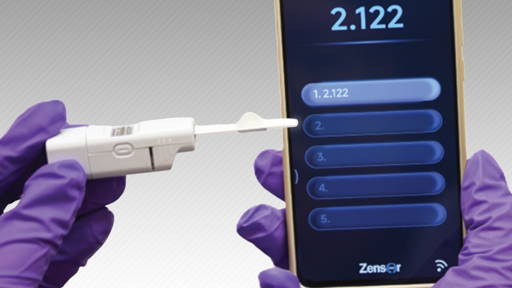
The system operates by preloading the catheter bag with reagents that emit fluorescent light when exposed to E. coli. Ultraviolet LEDs integrated into the sensor illuminate the urine sample, while a colour-sensitive detector measures fluorescence intensity. These data are transmitted wirelessly to a dedicated smartphone application.
The app functions as a remote interface, analytical dashboard and alert system. When bacterial levels exceed a defined threshold, the system automatically notifies the user, whether patient, caregiver or healthcare professional, enabling faster clinical decision-making.
Earlier detection
In laboratory-based testing using an in vitro bladder model, the sensor demonstrated the ability to detect E. coli at lower bacterial concentrations than conventional methods within three to nine hours. This represents a substantial reduction compared to the typical 24- to 48-hour timeframe associated with culture-based diagnostics.
According to lead researcher Dr. Weiming Xu, early detection could significantly reduce complications in catheter-dependent patients. “The goal is to enable faster medical response, reduce the risk of severe complications and potentially shorten hospital stays,” he noted.
While the current results are based on controlled laboratory conditions, the researchers emphasise that the next phase will involve testing with clinical samples to validate performance in real-world settings.
Remote infection surveillance
The project was conducted at the Center for Remote Health Technologies and Systems, part of the Texas A&M Engineering Experiment Station. The interdisciplinary team combined expertise in biomedical engineering, biosensing technologies and digital health systems.
Beyond detecting E. coli, ongoing research aims to expand the platform to identify additional pathogens. The team is also exploring the integration of similar biosensing technologies into intravenous catheters to monitor bloodstream infections.
From a digital health perspective, the innovation aligns with broader trends toward remote monitoring and smart medical devices. By embedding sensing technology directly into routine medical equipment, healthcare systems may move closer to continuous, data-driven infection surveillance.
If validated in clinical practice, smartphone-linked catheter sensors could complement existing diagnostics rather than replace them, providing an early warning layer that supports timely intervention. For hospitals seeking to reduce infection rates and improve patient safety, such technologies may offer a scalable, cost-effective addition to infection control strategies.